The rotamer preferences can be explained in term of hydrogen bonding.
The backbone nitrogene of N-cap, N1, N2, N3 are not involved in the
helical (i, i+4) backbone hydrogen bond, so specific bond with the side
chain occurs. (For example, the Capping box [Presta & Rose, 1988]). Here we
found specific hydrogen bonds in which the side chain of Ni bonds to the nitrogen backbone of Ni (i.e. MiSi) or Ni+1 (i.e. Mi+iSi).
Amino acids present different ability to make this bonds, Glu, Asp, Gln and Asn
making the best bonds. Following are the M2S2 and M3S2 hydrogen bonds in N2 which explain the prefrences for g-g+,
g+g- and g- rotamers.
|
|
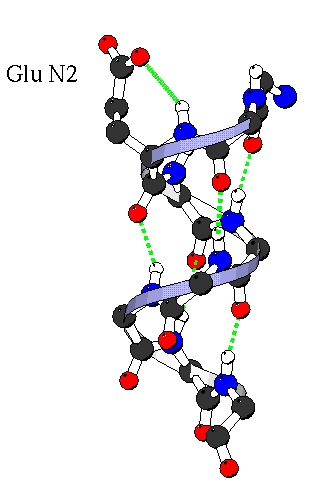
M2S2 with Glu in g- g+ . |
|
|
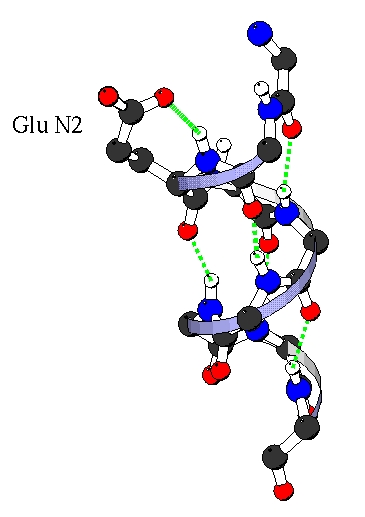
M2S2 with Glu in g+ g- . |
|
|
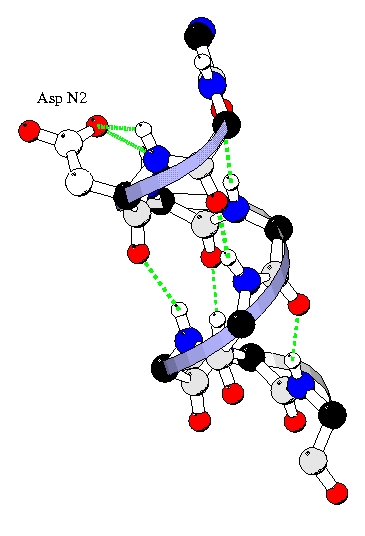
M2S2/M3S2 with Asp in g-. |